
2020
2020
83. Nickel Catalyzed Intramolecular 1,2‐Aryl Migration of Mesoionic Carbenes (iMICs)
A. Merschel, T. Glodde, B. Neumann, H.-G. Stammler, R. S. Ghadwal*
Angew. Chem. Int. Ed. 2020, n/a, doi: 10.1002/anie.202014328.
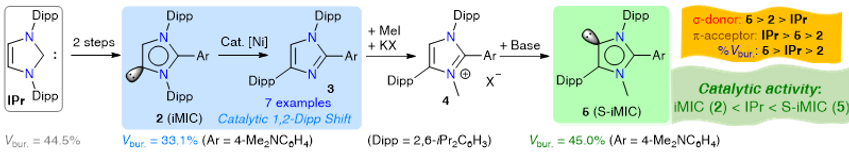
Intramolecular 1,2-Dipp migration of seven mesoionic carbenes (iMICAr) 2a-g (iMICAr = ArC{N(Dipp)}2CHC; Ar = aryl; Dipp = 2,6-iPr2C6H3) under nickel catalysis to give 1,3-imidazoles (IMDAr) 3a-g (IMDAr = ArC{N(Dipp)CHC(Dipp)N}) has been reported. The formation of 3 indicates the cleavage of an N‒CDipp bond and the subsequent formation of a C‒CDipp bond in 2, which is unprecedented in NHC chemistry. The use of 3 in accessing super-iMICs (5) (S-iMIC = ArC{N(Dipp)N(Me)C(Dipp)}C) has been shown with selenium (6), gold (7), and palladium (8) compounds. The quantification of the stereoelectronic properties reveals the superior σ-donor strength of 5 compared to that of classical NHCs. Remarkably, the percentage buried volume of 5 (%Vbur = 45) is the largest known amongst thus far reported iMICs. Catalytic studies show a remarkable activity of 5, which is consistent with their auspicious stereoelectronic features.
82. Isolation of a 16π-Electrons 1,4-Diphosphinine-1,4-diide with a Planar C4P2 Ring
D. Rottschäfer, B. Neumann, H.-G. Stammler, T. Sergeieva, D. M. Andrada,* R. S. Ghadwal*
Chem. Eur. J. 2020, xx, doi: 10.1002/chem.202003617.
Herein, we report the first 1,4‐diphosphinine‐1,4‐diide compound [(ADC Ph )P]2 ( 5‐Ph ) (ADC Ph = PhC{(NDipp)C}2 ; Dipp = 2,6‐iPr2C6H3) derived from an anionic dicarbene (ADC-Ph ) as a red crystalline solid. Compound 5‐Ph containing a 16π‐electron planar fused‐tricyclic ring system was obtained by the 4e reduction of [(ADC Ph )PCl2]2 (4‐Ph) with Mg (or KC8 ) in a quantitative yield. Experimental and computational results imply that the central 8π‐electrons C4P2 ring of 5‐Ph, which is fused between two 6π‐electrons C3N2 aromatic rings, is antiaromatic. Thus, each of the phosphorus atoms of 5‐Ph has two electron‐lone‐pairs, one in a p‐type orbital is in conjugation with the C=C bonds of the C4P2 ring, while the second resides in a σ‐symmetric orbital. This can be shown with the gold complex [(ADC-Ph )P(AuCl)2]2 (6‐Ph) obtained by reacting 5‐Ph with (Me 2S)AuCl. A mixture of 5‐Ph and 4‐Ph undergoes comproportionation in the presence of MgCl2 to form the intermediate oxidation state compound [(ADC-Ph)P]2(MgCl4) (7‐Ph), which is an aromatic species.

81. Saturated NHC Derived Dichalcogen Dications
D. Rottschäfer, D. E. Fuhs, B. Neumann, H.-G. Stammler, R. S. Ghadwal*
Z. Anorg. Allg. Chem. 2020, 646, 574–579.
Herein, the synthesis and characterization of dicationic di-halcogenide compounds [(SIPr)E]2(OTf)2 (3a-E) (E = S, Se, Te) based on a saturated N-heterocyclic carbene (NHC), SIPr [SIPr = C{N(Dipp)CH2}2, Dipp = 2,6-iPr2C6H3] are reported. Treatment of SIPr (1a) with elemental chalcogens affords the heavier ketone derivatives (SIPr)E (2a-E), which readily undergo oxidative E–E coupling reactions with triflic anhydride to yield the corresponding products 3a-E. Compounds 3a-E are air-stable crystalline solids and were characterized by NMR and UV/Vis spectroscopy as well as by X-ray diffraction methods.

80. Isolation of Elusive Electrophilic Phosphinidene Complexes with π-Donor N-Heterocyclic Vinyl Substituents
D. Rottschäfer, B. Neumann, H.-G. Stammler, D. M. Andrada, R. S. Ghadwal*

